The Wonders of Electromagnetism
How Cathodic Protection and Sacrificial Anodes Prevent Rusting

Iron, the most abundant metal on Earth, is extensively used in buildings, bridges, train cars, automobiles, and in everyday items. Modern civilization continues progressing on an extended trajectory that began during the Iron Age. However, iron is inherently plagued by the problem of rust. To shield iron from corrosion—particularly in underground and undersea structures—a technique known as cathodic protection is widely practiced. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial anode in place of iron.
Metals with strong ionization tendencies are utilized as sacrificial anodes
In chemistry, the tendency of a metal to become a cation (a positively charged ion) in water or an aqueous solution is defined in terms of its ionization energy. The degree of this tendency depends on the metal—some metals react with water at room temperature, while others react only with strong acids.
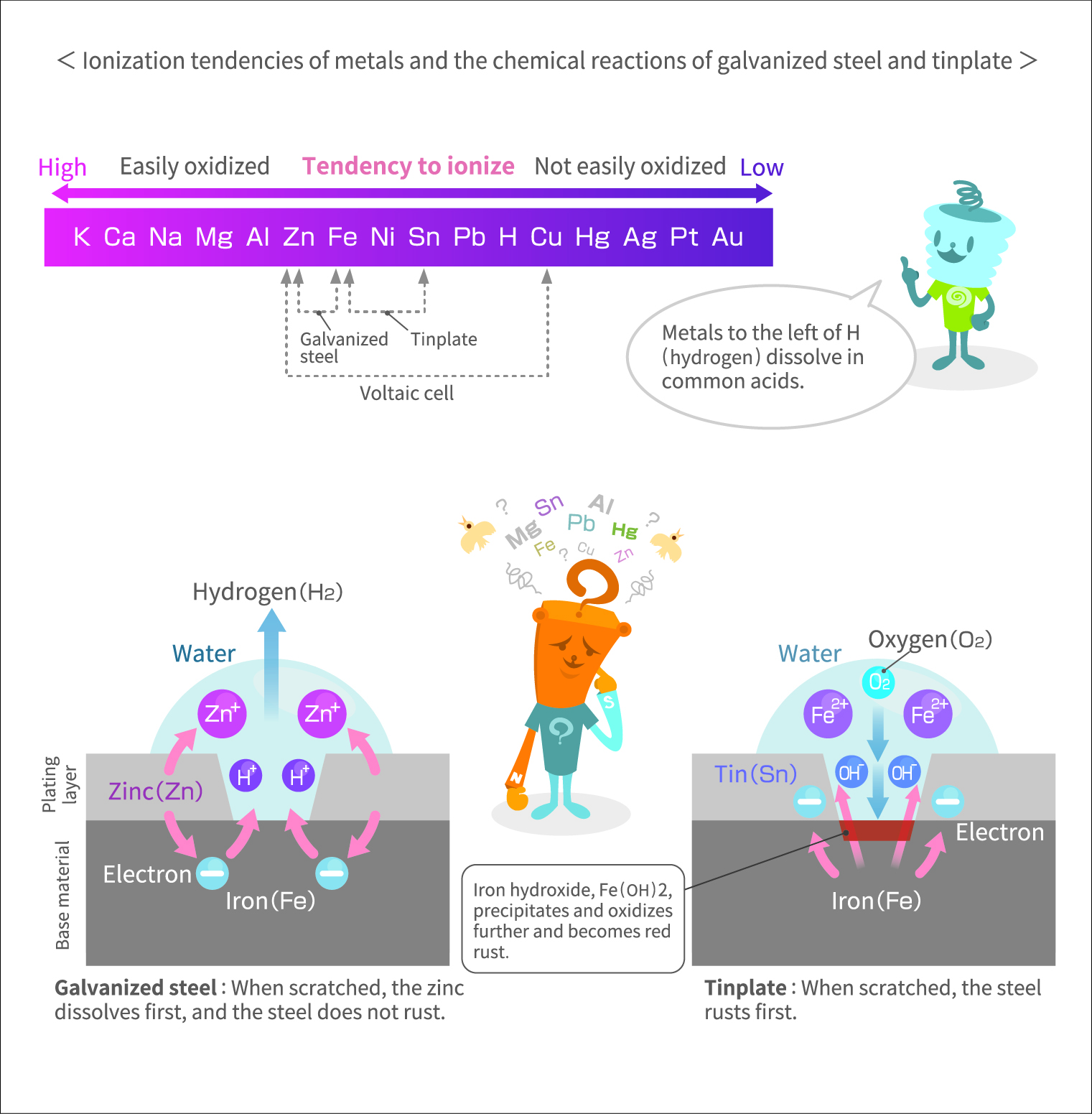
The following is a list of common metals arranged in descending order of tendency to ionize: potassium (K), calcium (Ca), sodium (Na), magnesium (Mg), aluminum (Al), zinc (Zn), iron (Fe), nickel (Ni), tin (Sn), lead (Pb), hydrogen (H), copper (Cu), mercury (Hg), silver (Ag), platinum (Pt), gold (Au). Metals positioned earlier on the list have a stronger tendency to ionize by releasing electrons, transforming into cations. They are more susceptible to oxidation and are stronger reducing agents (substances that “donate” electrons). Highly ionizable metals like potassium, calcium, and sodium are extremely reactive, requiring caution when handling. For instance, potassium reacts violently upon contact with water, producing a pale purple flame.
When a metal ionizes, it releases electrons (which are negatively charged), turning into a cation. The interaction between zinc and copper in an aqueous solution illustrates this phenomenon. Zinc, which has a higher ionization tendency than copper, dissolves into cations, and the released electrons flow toward the copper, creating an electric current. Harnessing this process created the world’s first battery, known as the voltaic cell.
Galvanized steel, produced by plating steel with zinc, is commonly used as a roofing material. It is a clever application of the ionization tendencies of two different metals. When scratched, the thin zinc coating easily reveals the underlying steel, exposing both metals together. Subsequent exposure to moisture, like raindrops, will cause the zinc to ionize instead of the iron in the steel due to zinc’s stronger tendency to ionize, preventing the steel from rusting. The scratches behave as local batteries: the zinc acts as a sacrificial anode that protects the steel against corrosion.
Tinplate is a material similar to galvanized steel. Tinplate, made by plating steel with tin, has been used in items like canned food containers and toys. It has a silver luster, but in damp conditions, rust forms on the iron because iron tends to ionize more easily than tin.
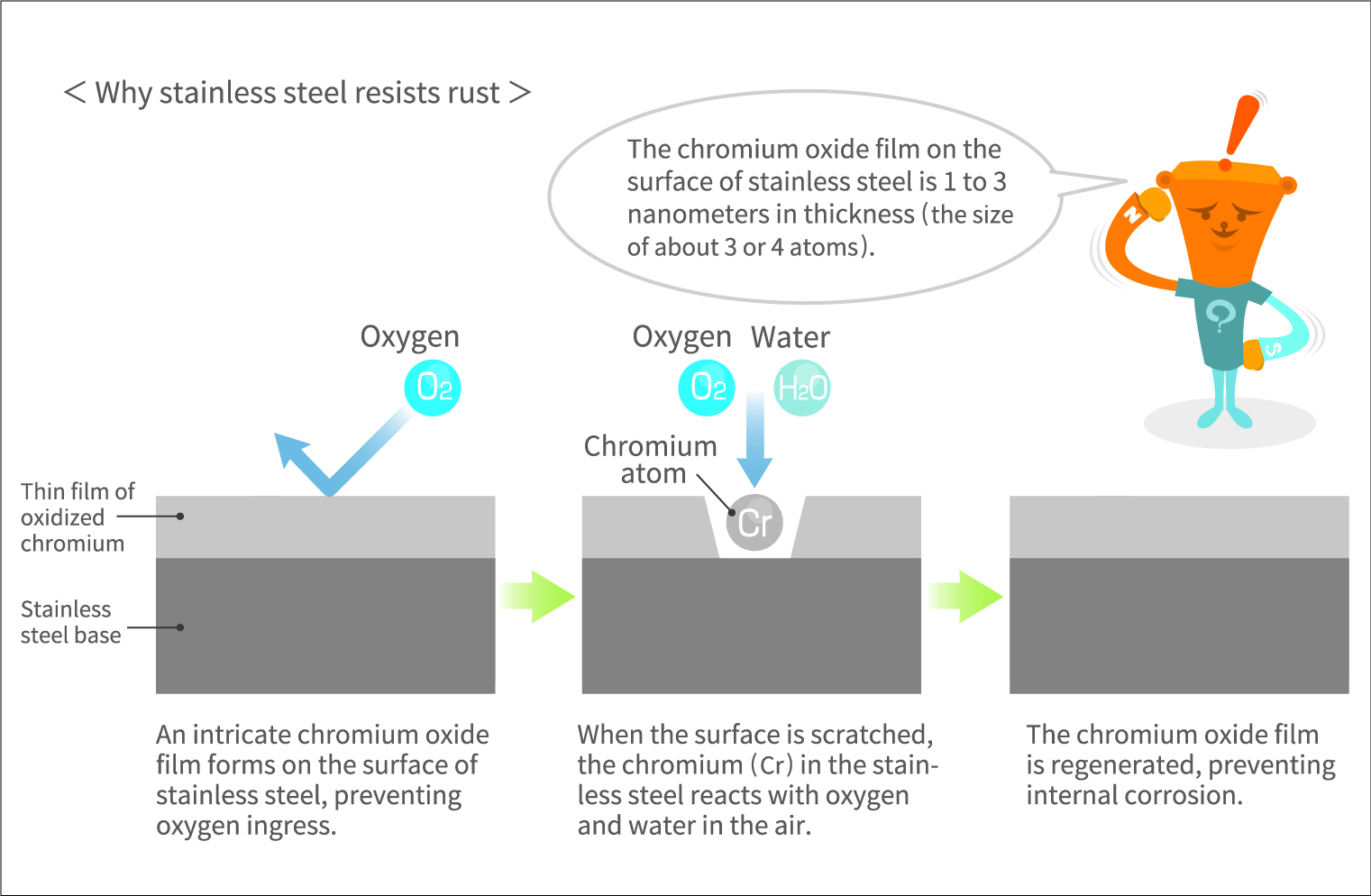
Stainless steel fights rust with rust
Stainless steel is considered one of the greatest inventions of the twentieth century. It is used everywhere, including household items like dishes and sinks, as well as various industrial products such as trains, vehicle exhaust systems, roofing and cladding materials in construction, and pipes and tanks in chemical plants.
Research into rustproof steel dates back to the nineteenth century with Michael Faraday. The legendary Damascus sword, well-known in the West for its rust resistance and remarkable sharpness, drove the young Faraday to unravel its mystery. He conducted his research by repeatedly melting various metals like chromium, nickel, and silver in crucibles to create alloy steels, ultimately developing the world’s first stainless steel. However, his formula required the addition of platinum, making it unsuitable for industrial use due to the expense.
Inspired by Faraday’s work, many scholars began delving into the study of steel alloys. Over time, it was discovered that adding a little above 10% of chromium makes steel resistant to rust. By the twentieth century, stainless steel was being produced industrially. The “18-8” marking, commonly found on items like tableware, indicates that the stainless steel contains 18% chromium and 8% nickel.
Chromium makes steel rust-resistant because it “fights rust with rust.” The chromium present in stainless steel reacts with substances like oxygen and water in the atmosphere, forming an extremely thin oxide film known as a passive film on the surface. This oxide film serves as a protective barrier, preventing further corrosion inward. Even when the surface of stainless steel is scratched, exposing the interior, the chromium immediately forms an oxide film, maintaining excellent corrosion resistance over extended periods of time. It is as if stainless steel possesses the ability to self-heal, akin to the skin of a living organism.
TDK’s technology plays an active role in cathodic protection
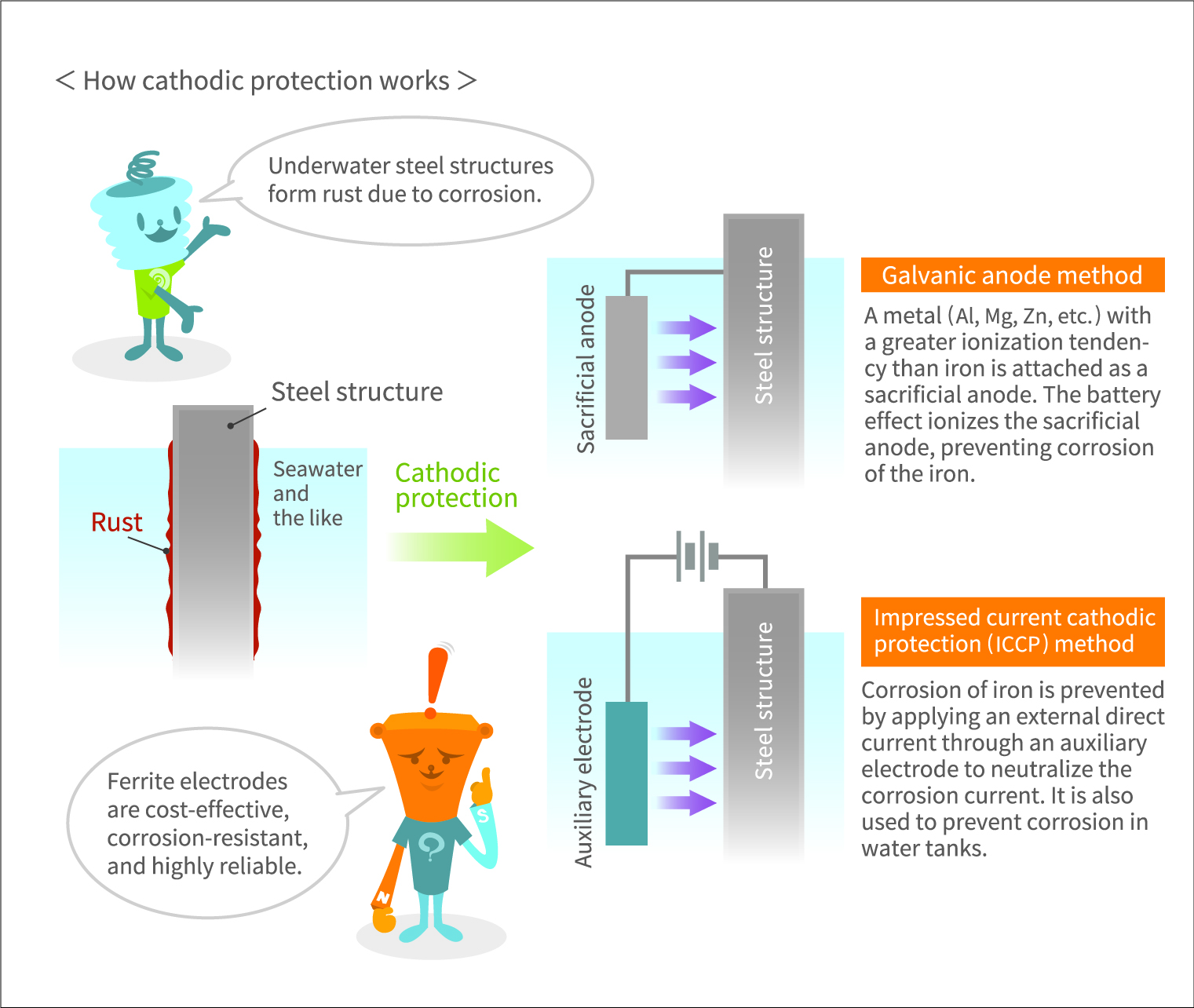
Steel structures in damp soil or seawater environments are susceptible to corrosion and rusting. Even in concrete structures, the rebar inside can develop rust. A technique known as cathodic protection is used to counteract such corrosion risks.
There are two commonly used forms of cathodic protection. The galvanic anode method involves attaching a sacrificial anode made of a metal with a greater ionization tendency than iron. Iron corrodes in an aqueous solution through the local battery effect, in which iron dissolves into cations, and the flow of the released electrons creates a corrosion current. By attaching electrodes like aluminum to underwater steel structures, the aluminum becomes a sacrificial anode in place of the iron in the steel, preventing the steel structures from corrosion. This is comparable to the process seen in galvanized steel, where the zinc acts as a sacrificial anode to prevent the steel from rusting.
The other method is impressed current cathodic protection (ICCP). In this approach, a direct current is applied from an external source in the opposite direction of the local battery effect occurring in the steel structures, neutralizing the corrosion current. The method is practiced in structures like harbor revetments and bridge girders. Cathodic protection also plays a critical role in chemical plants where corrosive chemicals are used because even stainless steel corrodes in such environments.
With ICCP, auxiliary electrodes are often used as anodes to carry the current. However, in a drinking water tank, for example, harmful metals dissolving out of the electrodes can contaminate the water. While a common solution is to use electrodes made of metals like titanium and platinum, ferrite is also a popular alternative. Ferrite, primarily composed of iron oxides, is cost-effective and exhibits robust corrosion resistance, ensuring high safety and reliability. TDK’s ferrite electrodes are manufactured from unique ceramic materials featuring uniform crystals and low resistance, offering excellent properties as electrodes. They are employed across a broad range of applications, including plating, surface treatment, wastewater treatment, and alkaline water ionizers.
Ferrite is subdivided into soft ferrite, found in components like transformer cores, and hard ferrite, used as a material to produce ferrite magnets. TDK’s ferrite magnets, in particular, offer some of the best characteristics in the world and are utilized in a wide variety of motors, including those for automobiles.
TDK is a comprehensive electronic components manufacturer leading the world in magnetic technology